Welcome to the Tenner Lab
Department of Molecular Biology and Biochemistry, Center for Immunology, University of California, Irvine, CA
Check out our lab news!
I am very honored to be the recipient of The Hans Muller-Eberhard Memorial Lecture Award. I am so appreciative of those who took the time and energy to nominate and support me for this distinction. My hope is that the existence of this award will inspire others to continue to explore the complement system and the opportunities it may provide to treat disease. Many individuals have contributed to the efforts of the laboratory over the years and to the excitement of discovery, facilitating the progress toward amelioration of inflammatory and degenerative diseases through both basic and preclinical research which hopefully will see translation to the clinic. My colleagues in various endeavors, at home and abroad, as well as students and trainees have informed me graciously, and I am so thankful to all.
Our Research Focus:
The Tenner laboratory is contributing to the emerging understanding of the role of the complement system in directing an appropriate immune response, with a specific focus on neurodegenerative conditions like Alzheimer’s disease (AD).
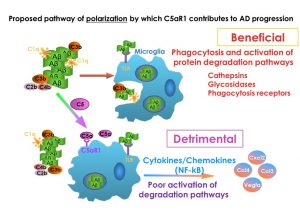
We are completing the preclinical studies necessary to establish rationale for a clinical trial testing an antagonist of the C5a receptor 1 as a treatment for AD and/or other acquired cognitive disorders caused by aging, cancer, neurodegeneration or other injury. In AD, the complement pathway (which is normally protective during infection) is activated by amyloid fibrils (and later by hyperphosphorylated tau) and can cause detrimental inflammation and neurotoxicity in an accelerating cycle. Administering a specific inhibitor of this inflammatory event improved cognitive performance and suppressed neuronal pathology in AD mouse models suggesting that this strategy may be a beneficial treatment to slow the progression of AD. We currently are defining the molecular pathways involved in these processes using bulk and single-cell RNA-Seq, immunohistochemistry, and behavior, and aligning our results to characteristics of human AD.
 Demonstrating the complexity of complement-mediated effects, others have shown that the early components of the complement cascade and a receptor expressed on microglia cells (the major scavenger cell of the brain) play a role in a normal process of synaptic pruning. Early in development, neurons have extranumerary or redundant synapses. Those that are rarely used or “weak” are eliminated via synaptic pruning or synaptic elimination. The mechanism of pruning is now being actively investigated by many laboratories during neuronal development, normal synaptic plasticity, and as a contributor to cognitive loss seen in neurodegenerative or neurological disorders. At least some pruning is complement-dependent, demonstrated by undesired or weak synapses that are tagged by complement components for recognition and uptake by microglia. This is beneficial during development, but whether induction of excessive pruning may contribute to detrimental effects in certain disorders in the adult, such as AD, is currently under investigation.
Demonstrating the complexity of complement-mediated effects, others have shown that the early components of the complement cascade and a receptor expressed on microglia cells (the major scavenger cell of the brain) play a role in a normal process of synaptic pruning. Early in development, neurons have extranumerary or redundant synapses. Those that are rarely used or “weak” are eliminated via synaptic pruning or synaptic elimination. The mechanism of pruning is now being actively investigated by many laboratories during neuronal development, normal synaptic plasticity, and as a contributor to cognitive loss seen in neurodegenerative or neurological disorders. At least some pruning is complement-dependent, demonstrated by undesired or weak synapses that are tagged by complement components for recognition and uptake by microglia. This is beneficial during development, but whether induction of excessive pruning may contribute to detrimental effects in certain disorders in the adult, such as AD, is currently under investigation.
We have also identified a novel neuroprotective pathway that is triggered by the complement component C1q (in the absence of the other complement components). C1q increases the response of the body’s scavenger cells to ingest dying cells and cellular debris and modifies the scavenger cell’s gene expression to promote an anti-inflammatory response, all of which are beneficial in the brain (as well as protective against autoimmunity). Furthermore, C1q is neuroprotective toward the toxic effects of amyloid fibrils. Thus, another current goal in the Tenner laboratory is to investigate these C1q-mediated neuroprotective pathways to enable therapeutic promotion of neuronal resilience.


